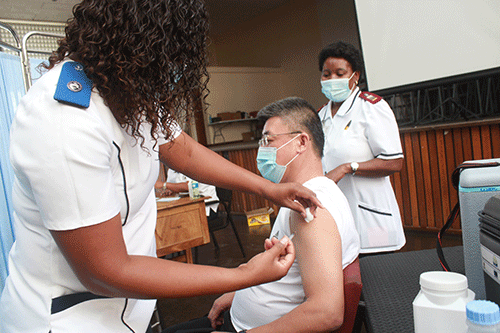
The judgement in a matter where the government is being sued for its alleged refusal to provide the public with information on the safety and possible adverse effects of Covid-19 vaccinations administered during the pandemic has been postponed.
On Wednesday, Deputy Judge President Hosea Angula postponed the ruling to 25 June. He said the postponement is a result of supplementary heads of arguments that the court requested from the parties. The documents were only filed on 31 May.
Angula will have to decide whether or not he will compel the government, President, health ministry, education ministry, Attorney General, and the Namibia Medicines Regulatory Council to provide their source of information and the information itself on which they relied on when they informed the public the Covid-19 vaccines were safe and effective for people with chronic illness and would help prevent serious illness, hospitalisation, and death from Covid-19 infection as requested by Health Defence League (HDL).
The HDL, which represents 537 people along with members of the public Monika Ruppel, Manfred Jochen Förtsch, Werner Gertz, and Paul Du Plessis further wants the court to declare that the government’s decision to permit Covid-19 vaccine, Pfizer/BioNTech to be administered to the public while it was not registered in terms of the Medicines and Related Substance Control Act was unlawful.
Furthermore, the court must declare null and void any indemnification forms that the group might have signed before getting vaccinated.
The group claims that the suit is not about whether vaccination is good or bad, it is about the right to receive information to make informed choices, and the government’s duty to make such information available to the public.
However, the government believes that it has a fiduciary duty as a recipient of information imparted and received in confidence not to disclose such information unless they are permitted by the vaccine manufacturers.
Furthermore, the group’s intent to give rise to a debate on the efficacy of Covid-19 vaccines, which goes far beyond the Namibian borders and challenge the country’s policy on the administration of Covid-19 vaccines.
According to the government, all the information requested by the group are readily available publicly and some have been published by the World Health Organisation and the health ministry in notices.
In addition, the approach to vaccination selection in Namibia was founded on the principle of evidence-based medicine, putting in place stringent guidance for the evaluation of data emerging from clinical trials in support of issuing vaccine specific recommendations.
The group is represented by Raymond Heathcote with Tinashe Chibwana representing the government.
-mamakali@nepc.com.na